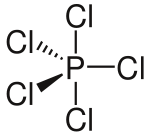Bent Out of Shape
Problem
When a molecule consists of one central atom with several others covalently bonded to it the electrons in these bonds repel each other. The result of this is that the bonded atoms will be oriented as far away from each other as possible, eg.
![]()
The angle formed between the two oxygen atoms (the "bond angle") is $180^\circ$ - given that the length of the bonds are fixed the oxygen atoms could not be further apart.
Here, the central boron has three fluorine atoms bonded to it. The angle between any two fluorines is the same and it is $120^\circ$ . Again, the three F atoms could not have arranged themselves to be further apart.
This occurs because the bonds are formed of pairs of electrons, which carry a negative charge. If two bodies (in this case bonds) are negatively charged they will repel each other.
Draw diagrams to represent the structures of the following molecules. Can you work out what the bond angles are? (You don't need to know anything about the molecules!)
NH$_{4}^{+}$
CH$_{4}$
BeH$_{2}$
AlCl$_{3}$
PCl$_{5}$
SF$_{6}$
Water (H$_{2}$O) has a bond angle of $104.5^\circ$. What would you have expected the bond angle to be? Why do you think it might be different?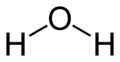
Similarly, NH$_{3}$ has a bond angle of $107.5^\circ$. As is the case for water, this is because the nitrogen atom has an unbonded pair of electrons in its outer shell - a lone pair. This acts much like a bond, repelling the electrons in the covalent bonds. Why do you think the bond angle is not the same as for NH$_{4}$? Draw the two molecules side by side. How does the orientation of the
hydrogen atoms differ? How many covalent bonds is the lone pair here equivalent to in terms of repulsive effect? Is this the same as for the lone pairs in water? Does this surprise you?
Getting Started
If you are having trouble picturing how these molecule might look, try using blu tack and string to make models of the atoms and their bonds.
If you're still stuck, scroll down on this page to see diagrams of the molecules.
For the question about water, it will help you to think about the electrons in oxygen. How many valence electrons does oxygen have? What happens to the valence electrons that don't get used in bonding?
Structures of molecules given:
You will notice that in some of these diagrams some bonds are shown as normal lines, some as thick lines and some as dashed lines. This says nothing about the chemical nature of the bonds but describes their orientation in three dimensions. A normal line indicates a bond in the plane of the page (or screen); a thick line indicates a bond coming out of the page and a dashed line indicates a bond
going into the page. If this is confusing, don't worry! Try using these diagrams to help you make a model of the molecules or ask your teacher to explain further.
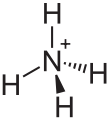
CH$_{4}$ has the same structure as NH$_{4}^{+}$
![]()
AlCl$_{3}$ has the same structure as BF$_{3}$