Or search by topic
Number and algebra
Geometry and measure
Probability and statistics
Working mathematically
For younger learners
Advanced mathematics
Pack Man



- Problem
- Student Solutions
Atoms can be thought of as hard spheres of a fixed radius, for many purposes in physics.
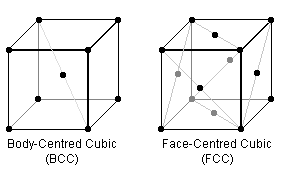
In the BCC & FCC crystal lattice arrangements (the repeating cube of each is pictured above), certain planes are "close packed" i.e. the "hard spheres" are thought of as touching. Steels can have either packing structure, but are generally structurally better in the more densely-packed form which, for example, don't become brittle at low temperatures.
To begin, visualise the crystal arrangements above where the black dots represent the centre of identical spherical atoms of fixed radii which just touch each other.
Can you tell which of BCC or FCC is this better, more dense form? Can you prove it?
Pure copper is structured in the FCC form, has atomic mass 63.5, and atomic radius 128 picometres. Estimate the density of copper. How does your result compare with the actual density of copper?

