Copyright © University of Cambridge. All rights reserved.
'pH Temperature' printed from https://nrich.maths.org/
Show menu
The initial key to this problem is to realise that for self-dissociating water, [H]$^+$ = [OH]$^-$.
Therefore, K$_W = [H^+]^2$
Since pH = $-log_{10}[H^+]$
$\mathbf{\Rightarrow K_W = 10^{-2pH}}$
When pH = 7; $K_W = 1 \times 10^{-14}$
pH =6.8; $K_W = 2.51 \times 10^{-14}$
pH = 7.2; $K_W = 0.398 \times 10^{-14}$
Plotting a graph of $K_W$ versus temperature gives:
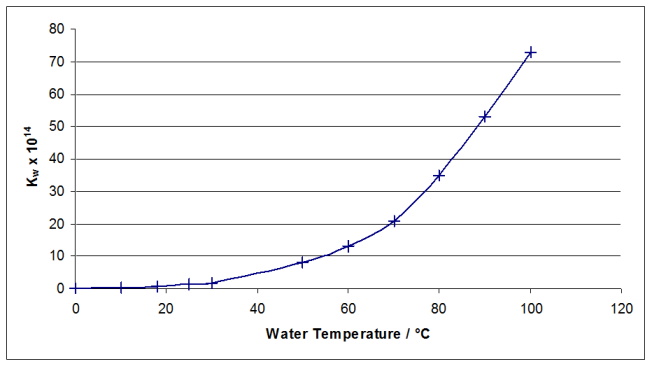
By drawing a smooth curve of best fit through the points, the relevant temperatures can be read off the graph for the given values of $K_W$. If you draw the graph for the entire temperature range, it is difficult to read off accurate values in the required range, which are all smaller than $10\times 10^{14}$. Plotting the curve through the first three points allows us to read off accurate values.